
Physics of energy sources
 Before we speak about energy, it is useful to lay some groundwork to
understand it better. Most people, when talking about energy sources, are
really talking about energy carriers. Think about gasoline, coal, ethanol,
water, wind. Where does their energy actually come from?
Before we speak about energy, it is useful to lay some groundwork to
understand it better. Most people, when talking about energy sources, are
really talking about energy carriers. Think about gasoline, coal, ethanol,
water, wind. Where does their energy actually come from?
The conservation of energy law and the first law of thermodynamics state that energy cannot be created or destroyed. It can only change form. Einstein showed that mass and energy are equivalent, leading to the famous equation E=mc².
So physically, there are no "sources" of energy, because it doesn't just come into existence. It just looked that way to us before we understood what energy is. What we see as an energy source is just energy being taken from a carrier and transformed from one sort into another. But since the use of the word "source" is so ingrained, I'll not confuse you be being a stickler for accuracy.
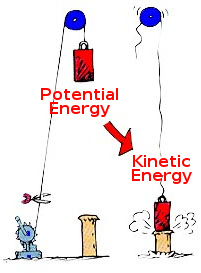 While it is possible for most kinds of energy to be transformed into other
kinds without loss (e.g. dropping something converts potential energy into
kinetic energy perfectly), it is impossible to convert thermal energy into
other forms with 100% efficiency. This is usually called the
second law of thermodynamics; systems tend to evolve towards larger
entropy.
While it is possible for most kinds of energy to be transformed into other
kinds without loss (e.g. dropping something converts potential energy into
kinetic energy perfectly), it is impossible to convert thermal energy into
other forms with 100% efficiency. This is usually called the
second law of thermodynamics; systems tend to evolve towards larger
entropy.
In mechanical systems, friction usually consumes a part of the energy put into the system, and dissipates it as heat. That is why machines are not 100% efficient. The ubiquitous ball bearing uses rolling resistance to reduce friction. But by using fluid bearings it is possible to reduce friction considerably more. Small machines are usually less efficient than big ones.

This work
is licensed under a Creative Commons
Attribution 3.0 Unported License.
| This post is part of our Energy Primer lunchtime series.
Read all of the entries: |
Want more in-depth information? Browse through our books.
Or explore more posts by date or by subject.
About us: Anna Hess and Mark Hamilton spent over a decade living self-sufficiently in the mountains of Virginia before moving north to start over from scratch in the foothills of Ohio. They've experimented with permaculture, no-till gardening, trailersteading, home-based microbusinesses and much more, writing about their adventures in both blogs and books.
Want to be notified when new comments are posted on this page? Click on the RSS button after you add a comment to subscribe to the comment feed, or simply check the box beside "email replies to me" while writing your comment.
- Remove comment
- Remove comment
