
Soil cations
 Before I go further into
Solomon's remineralization strategies, you have to know more about
those minerals I mentioned vaguely in my
leaching post.
Although there are a slew of minerals plants use in large and small
quantities, Albrecht (and those who have built on his work) are
primarily interested in the calcium (Ca), magnesium (Mg), potassium
(K), and sodium (Na). Albrecht believed that prairie soils
provide optimal crop nutrition, so he suggested we mimic the ratio
found there: 68:12:4:2. Other practitioners have suggested
slightly different values, such as 62:18:4:2 for sandy soil, or
85:5:2-4:1-2 if you're following the work of Victor Tiedjens and think
calcium is of most interest.
Before I go further into
Solomon's remineralization strategies, you have to know more about
those minerals I mentioned vaguely in my
leaching post.
Although there are a slew of minerals plants use in large and small
quantities, Albrecht (and those who have built on his work) are
primarily interested in the calcium (Ca), magnesium (Mg), potassium
(K), and sodium (Na). Albrecht believed that prairie soils
provide optimal crop nutrition, so he suggested we mimic the ratio
found there: 68:12:4:2. Other practitioners have suggested
slightly different values, such as 62:18:4:2 for sandy soil, or
85:5:2-4:1-2 if you're following the work of Victor Tiedjens and think
calcium is of most interest.
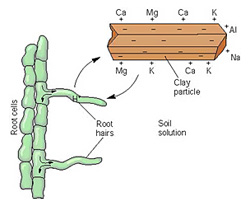
If you remember high
school chemistry, you may recall that these four minerals are all
cations, meaning they're positively charged and are attracted to
negatively-charged clay and humus particles. The amount of
negatively charged spots in the soil isn't unlimited, though, so
there's only so much space to go around (measured as the cation
exchange capacity),
and all cations don't cling equally tenaciously to the spots that are
available. Calcium is strongest, followed by magnesium,
potassium, and sodium, in that order, so if you dump masses of lime
(calcium) in the soil, calcium will fill all of the spots, bumping off
the weaker cations. Meanwhile, if you only add a moderate amount
of calcium, only the lowest cations on the totem pole will be bumped
off, so you'll end up with a higher ratio of calcium and a lower ratio
of sodium in the soil.
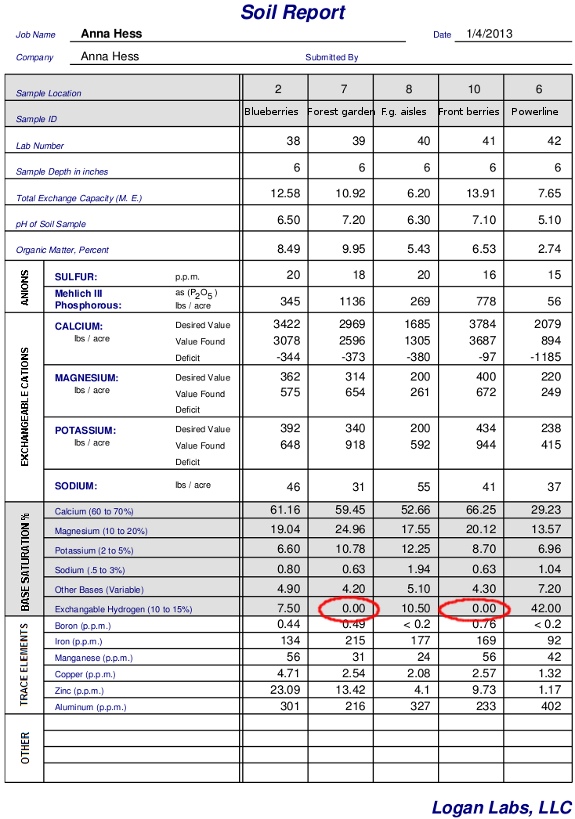
Even though we don't
talk about it much, the lowest cation on the totem pole is actually
hydrogen, which is pulled out of water to fill empty spots if there
aren't enough other cations to go around. If hydrogen has to fill
any negatively charged spots in your soil, that means you're soil is
acidic. Take a look at my soil test results above and notice that
the two samples without exchangeable hydrogen (circled in red) are also
the samples where the pH is greater than 7, meaning
the soil is alkaline instead of acidic.
This is also why adding
lime to your soil raises its pH. The extra calcium in lime knocks
hydrogen ions back into solution where they merge with the carbonate
ions from the lime to create water and carbon dioxide. Without
those extra hydrogen ions hanging around, the soil is no longer acidic.
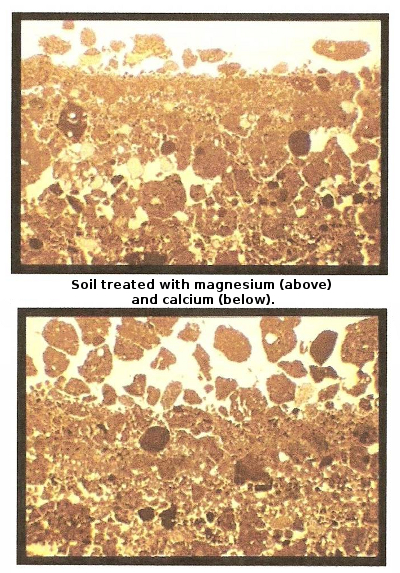 The reason I've spent so long
on this chemistry is because it explains how you can change the ratio
of cations in your soil. Adding a stronger cation creates a
cascade of other cations being kicked off the negatively charged spots
in soil, and as long as you add lots of water to leach the unattached
cations from the water, those cations disappear into the subsoil and
leave the topsoil different than you found it. This can be handy
if you garden on clay (like I do) and if your soil contains too much
magnesium (like mine does), which tightens soil up and makes it
waterlogged. Those of you gardening near the coast who irrigate
with water high in salts will also find this handy since excess sodium
can be even more harmful to soil than excess magnesium since high
sodium levels tighten soil even more and can also be toxic to plants.
The reason I've spent so long
on this chemistry is because it explains how you can change the ratio
of cations in your soil. Adding a stronger cation creates a
cascade of other cations being kicked off the negatively charged spots
in soil, and as long as you add lots of water to leach the unattached
cations from the water, those cations disappear into the subsoil and
leave the topsoil different than you found it. This can be handy
if you garden on clay (like I do) and if your soil contains too much
magnesium (like mine does), which tightens soil up and makes it
waterlogged. Those of you gardening near the coast who irrigate
with water high in salts will also find this handy since excess sodium
can be even more harmful to soil than excess magnesium since high
sodium levels tighten soil even more and can also be toxic to plants.
Solomon's pet peeve has
more to do with nutrient-density than with soil texture, though.
He's found that leached soils are usually excessively high in
potassium, especially if you add hay, straw, or wood products to your
garden as compost or mulch. High potassium levels make plants
grow fast, but the produce is low in protein, is high in carbohydrates,
and is usually deficient in calcium and phosphorus. Livestock who
eat plants grown on high-potassium soil gain weight but don't breed
well, and people who eat from that type of soil tend to gain weight and
have health problems as well. Stay tuned for more details on how
to rebalance your soil for optimal nutrition.
This
post is part of our The Intelligent
Gardener lunchtime series.
Read all of the entries:
|
Want more in-depth information? Browse through our books.
Or explore more posts by date or by subject.
About us: Anna Hess and Mark Hamilton spent over a decade living self-sufficiently in the mountains of Virginia before moving north to start over from scratch in the foothills of Ohio. They've experimented with permaculture, no-till gardening, trailersteading, home-based microbusinesses and much more, writing about their adventures in both blogs and books.
Want to be notified when new comments are posted on this page? Click on the RSS button after you add a comment to subscribe to the comment feed, or simply check the box beside "email replies to me" while writing your comment.

It's even a little bit more complex than you've decribed: the active transport enzyme systems that get the cations into the cells often operate on an exchange (Na-K or Ca-Mg) &/or competitive basis, so ratios may be as important as total concentrations.
To say that prairie soil is healthiest is a bit of a tautological argument: the plants growing there naturally have adapted to make most efficient use of the ambient environment. To extrapolate that to say that crop species would benefit from natural prairie conditions may or may not be justified.
Your final comments about health effects of soil chemistry doesn't pass close critical scrutiny, although it is true that performance (ie- wt gain) of beef cattle has been shown to be related to mineral ratios in their feed.